NANOCELLE® CANNABIS
Background
Medlab’s work revolved around 3 cannabinoid formulations:
A dominant CBD with THC (known as NanaBidial)
A CBD-only formulation (known as NanoCBD)
An equally weighted CBD and THC (known as NanaBis).
Projects NanoCBD and NanaBis were earmarked for new drug applications in the stress/anxiety and cancer pain markets; with NanaBis commanding the majority of the clinical work, and although the work to support the new drug application is unfinished, the data is compelling.
NanoCBD and NanaBis depending on the formulations, utilised both standardised botanical material and synthetic markets with US FDA DMF’s.
All three formulations at some point were made available to Australian patients for ethical use under the ARTG Special Access Scheme (SAS). Today NanaBis and NanoCBD are still available under the ARTG SAS from a 3rd party - Please note MEDLAB does not supply or market to consumers, MEDLAB doesn’t sell cannabis to the public. MEDLAB licences its intellectual property (IP) to 3rd parties for inclusion into their product offering.
The Data
The Studies - ethics approved and published
Case Studies - ethics approved and published
Key Summary points:
Published work into NanoCelle® Cannabinoids can be found here.
The NanoCelle® use in the cannabinoid projects has been extensive, in the accumulation of data NanoCelle® cannabis via:
Pharmacokinetic (PK), ethics approved - healthy
Pharmacokinetic (PK), ethics approved - unhealthy
Phase 2 ethics approved, Hospital in/out-patient safety, tolerability, and efficacy trial
RWE, ethics approved, safety, tolerance, and longevity
The data shows the following:
NanoCelle® cannabis is safe
longevity data across ~1200 patients showed continued tolerable use for an average of 5.5 months
all doses were titrated up to achieve tolerance
Opioid sparring was demonstrated within 72 hours of commencing a NanoCelle CBD and THC formulation ( specifically)
Adverse events showed 1% SAE, of which 90% was expected, no sequelae, 9% mild to moderate adverse events, and 80% expected. No reported instance of CARPA.
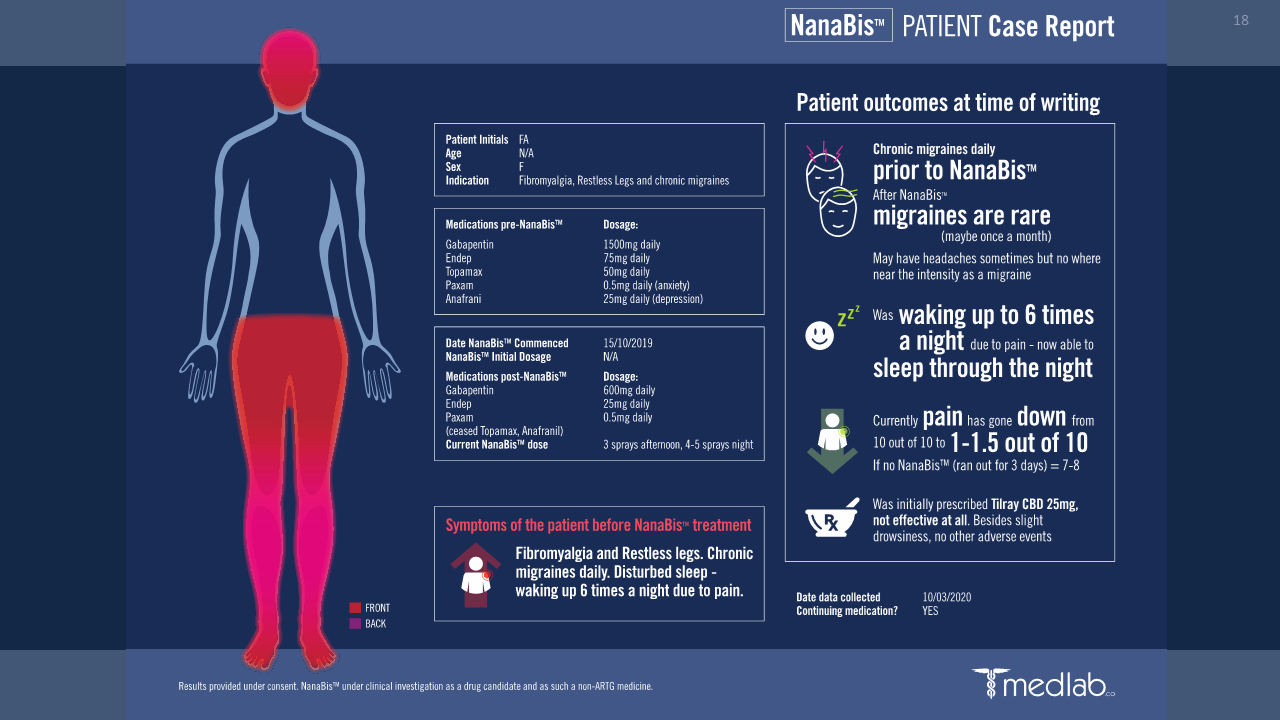
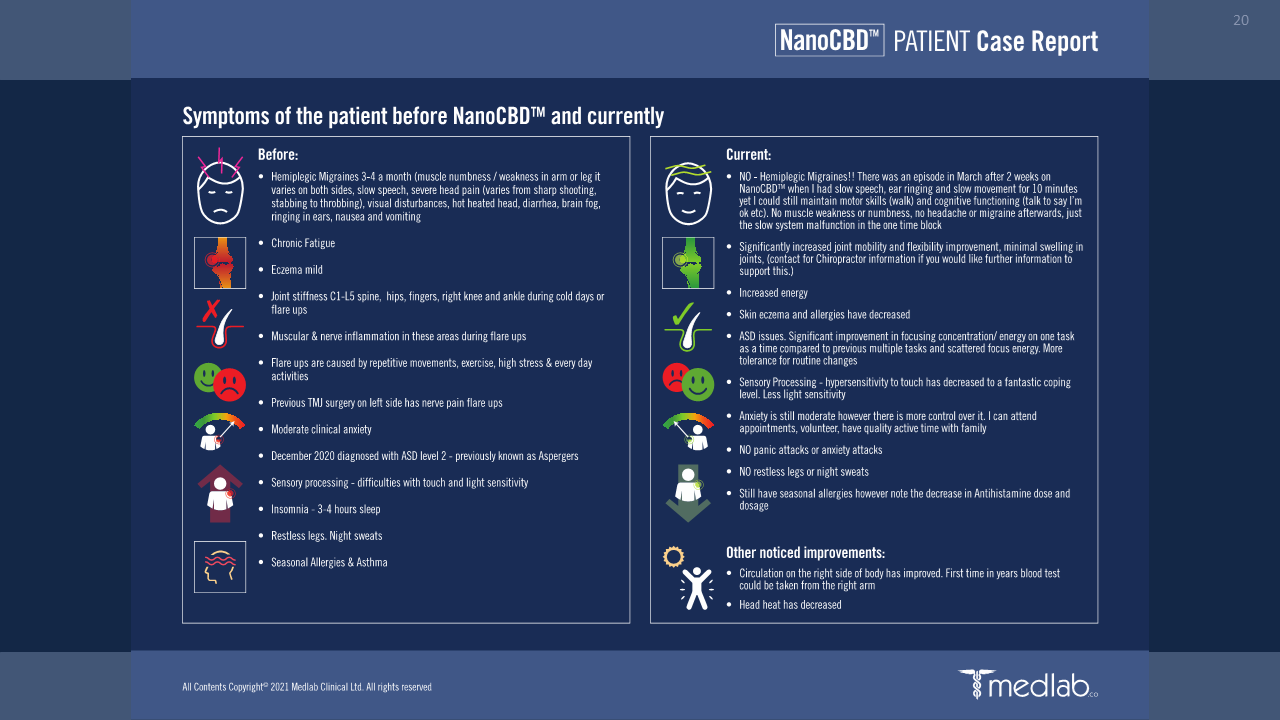
Improvements in quality of life (QoL) especially in sleep, eating, mobility, and cognitive function.
Improvements in pain tolerance.